Blog
Crystal Fields Of Ketamine
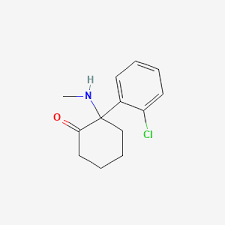
Crystal Fields Of Ketamine, Ketamine, a compound initially developed as an anesthetic, has gained renewed attention in recent years due to its potential in treating depression, PTSD, and chronic pain. While much of the focus has been on its pharmacological effects, understanding the crystal fields of ketamine opens a fascinating window into its chemical nature and structural behavior.
What Are Crystal Fields?
In chemistry and solid-state physics, “crystal field” refers to the effect of the surrounding electric fields created by neighboring ions or molecules on a central atom or ion, typically in a crystalline lattice. This concept, known as Crystal Field Theory (CFT), is primarily applied to transition metal complexes, but it can also help interpret molecular and crystalline behaviors in organic compounds like ketamine.
The Molecular Structure of Ketamine
Ketamine’s chemical name is (RS)-2-(2-chlorophenyl)-2-(methylamino)cyclohexanone. It belongs to the arylcyclohexylamine class and exists in two enantiomeric forms: S-(+)-ketamine and R-(–)-ketamine. In its solid state, ketamine forms crystalline structures depending on the solvent used during crystallization and the form (free base or hydrochloride salt).
Crystal Fields of Ketamine: Structural Behavior
In solid-state studies, the crystal fields of ketamine can refer to:
-
Intramolecular interactions within the crystal lattice that influence molecular conformation.
-
Hydrogen bonding and Van der Waals forces, which determine how ketamine molecules align in the crystal.
-
Polymorphism, where ketamine can crystallize in different forms depending on temperature, humidity, and solvents.
These structural characteristics are critical when producing pharmaceutical-grade ketamine. Different polymorphic forms can affect bioavailability, stability, and solubility, all of which impact therapeutic effectiveness.
Importance in Pharmaceutical Applications
The crystal fields of ketamine aren’t just academic curiosity—they play a key role in drug formulation. For instance, ketamine hydrochloride, the most common pharmaceutical form, exhibits specific crystalline properties that ensure stability and reproducibility in dosing.
Additionally, understanding how ketamine’s crystals behave at the molecular level aids in:
-
Developing extended-release formulations
-
Designing intranasal and sublingual delivery systems
-
Preventing degradation or loss of potency during storage
Research Implications and Future Directions
As researchers develop novel formulations like esketamine nasal sprays or ketamine lozenges, manipulating the crystal fields of ketamine becomes increasingly important. Advanced techniques like X-ray diffraction (XRD) and Fourier-transform infrared spectroscopy (FTIR) are employed to analyze crystal morphology and ensure consistent quality.
Moreover, knowledge of ketamine’s crystalline behavior may lead to new analogs or derivatives with improved therapeutic profiles or fewer side effects.
Conclusion
The crystal fields of ketamine reveal a deeper layer of understanding beneath its well-known pharmacological effects. Through insights into its crystalline structure, scientists can better control its physical properties, paving the way for more effective and safer applications in medicine. As ketamine continues to evolve as a powerful tool in mental health treatment, its crystal chemistry remains a cornerstone of innovation and discovery.
